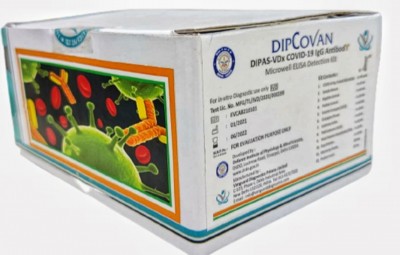
Chennai, With the 35 years old Rs 400 crore medical syringes brand Dispovan and the Defence Research Development Organisation's (DRDO) Dipcovan - coronavirus antibody detection kit - sounding similar, Hindustan Syringes & Medical Devices Ltd will request the defence lab to have a different brand name, said a top company official.
"We will politely request DRDO to go in for a different name for the coronavirus antibody detection kit as the brand name Dipcovan sounds very similar to that of our 35 years old medical syringe brand Dispovan," Rajiv Nath, Managing Director, Hindustan Syringes, told IANS.
Nath said though both the products are different in nature, they are classified as medical devices and hence there will be a confusion at the marketplace.
He said the confusion would happen as a private company will be manufacturing and marketing the Covid-19 antibody detection kit and people may associate the product as belonging to the house of Hindustan Syringes, the makers of Dispovan brand syringes.
According to Nath, the Dispovan syringe sales are around Rs 400 crore and the brand stakes are high for the company.
He hoped the DRDO will consider his request favourably and quickly.
On Friday, DRDO said one of its labs, the Defence Institute of Physiology and Allied Sciences (DIPAS) along with Vanguard Diagnostics Pvt Ltd has developed an antibody detection-based kit Dipcovan.
Perhaps the brand name Dipcovan was arrived at by combining DIP from DIPAS, Co from Covid and Van from Vanguard Diagnostics.
According to the DRDO, the kit can detect both spike as well as nucleocapsid (S&N) proteins of SARS-CoV-2 virus with a high sensitivity of 97 per cent and specificity of 99 per cent.
The antibody detection kit is approved by Indian Council of Medical Research (ICMR) in April 2021, DRDO said.
In May 2021, the product received the regulatory approval from the Drugs Controller General of India (DCGI), Central Drugs Standard Control Organisation (CDSCO), Ministry of Health and Family Welfare, to manufacture for sale and distribution.
The New Delhi based Vanguard Diagnostics will commercially launch the product during the first week of June 2021.
Readily available stock at the time of launch will be 100 kits for about 10,000 tests with a production capacity of 500 kits/month post launch.
It is expected to be available at about Rs 75 per test.


.jpeg)

